orange book pharmacy codes
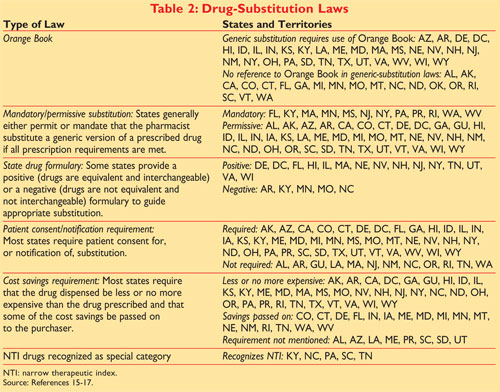
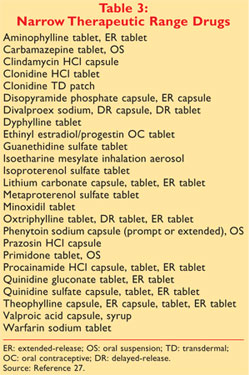
An A designation means that the FDA considers the drug to be the therapeutic equivalent of another pharmaceutically equivalent drug. In Conclusion Generic drugs now represent 88 percent of drugs dispensed in the United States.
The second letter A B C D E N O P R S T or X.
. Controlled substances maintenance drugs FDA Orange Book codes for multisource products and Healthcare Common Procedure Coding System HCPCS J-codes for reimbursement of injectable drugs Current NDC numbers. What is the purple book. 5 rows 17 In previous editions of the Orange Book FDA provided a chart outlining therapeutic.
Order Optum360 Coding Books Online Now. ICD-10 CPT HCPCS DRG More. The Orange Book uses Therapeutic Equivalence codes TE codes a short series of letters and sometimes numbers eg AB AB2 BX to categorize drugs based upon their assessed equivalency.
A guide to community. The FDA ORANGE BOOK Before discussing the specific meaning of each of the Orange Book TE codes a few definitions are in order. The Orange Book is available as a PDF in print and electronically.
Ad Order Online Save On Optum360 Essential Code Books. Orange Book Codes The Orange Book Codes supply the FDAs therapeutic equivalence rating for applicable multi-source categories. The Orange Book Introduction.
- by DEA Drug Code Number - DEA CSA SUBSTANCE NUMBER SCH NARC OTHER NAMES Codeine preparations - 200 mg100 ml or 100 gm V Y CosanylRobitussin A-CCheracolCerosePediacof Difenoxin preparations - 05 mg25 ug AtSO4du V Y Motofen Dihydrocodeine preparations 100mg100 ml or 100 V Y Cophene-S various others gm. Their guides are available by subscription or individual purchase. CSA 7458 9663 4000 4000 Controlled Substances - Alphabetical Order - DEA SUBSTANCE NUMBER SCH NARC OTHER NAMES 1-4-Fluorobenzyl-1H-indol-3-yl2233- 7014 I N FUB-144.
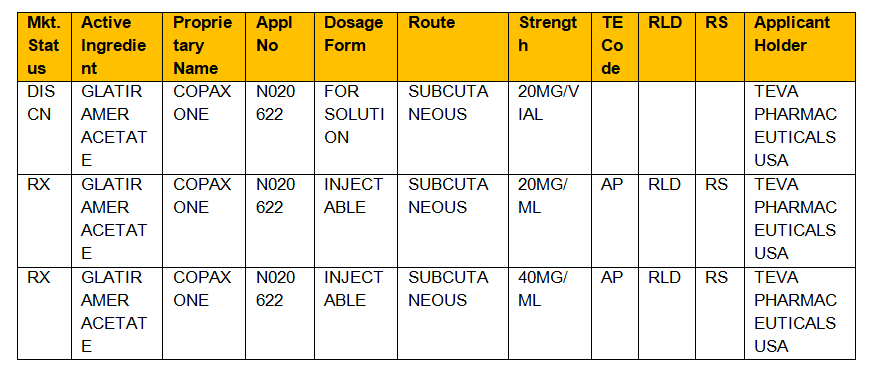
Trusted for more than 120 years RED BOOK includes pricing information on over 300000 prescription and over-the-counter pharmaceuticals chemicals and medical devices and supplies available on the web or in flat data files. Approved Drug Products with Therapeutic Equivalence Evaluations. Various codes and their interpretations are described in table 1.
Reduce confusion with NDC numbers that are maintained in 5-4-2 formats. In addition the Orange Book contains therapeutic equivalence evaluations 2 character rating codes for approved multisource prescription drug products generic drugs. Save 55 On 2022 Editions.
The Purple Book is a compendium of FDA-approved biological products and their biosimilar and interchangeable products. E 2XELOS is a company part owned by the UK government. Basics in drug approval process with reference to the Orange Book Presented by.
1xecutive Agencies Non Departmental Public Bodies and Non Ministerial Departments. On March 23 2020 FDA removed from the Orange Book the listings for biological products that have been approved in applications under section 505 of the FDC Act because these products are no longer listed drugs see section 7002 e 4 of the Biologics Price. The electronic availability of the Orange Book brings this valuable tool to the web for healthcare.
1 Need of the Orange Book Definition Introduction to 2 History 3 the Orange Book Objectives 4 3 Contents of the Orange Book 5 18 4 Cumulative Supplement 19 5. Is particularly critical in. Sumanta Mondal_MPhar m 1 th Sem.
If a number changes the database supplies both the. It is the publication of. Rucha Pathak Roll No.
Codes beginning with B indicate bio-equivalence has not been confirmed. The first letter indicates that the FDA has either concluded a generic formulation is therapeutically equivalent to the reference drug an A Code rating or that the compared drugs. Corporate Governance Code 2 requirements and overseeing the preparation of the governance.
Every drug listed in the Orange Book has a 2-letter code. 3 rows FDAs orange book and ab ratings of pharmaceutical drug products. The publication Approved Drug Products with Therapeutic Equivalence Evaluations commonly known as the Orange Book identifies drug products approved on the basis of safety and effectiveness by.
There are ASCII text files of the Orange Book drug product patent and exclusivity data at the Orange Book Information Data Files page. A-rated drugs are those which the FDA considers to be therapeutically equivalent and therefore substitutable where permitted by the prescriber. The electronic version of the Orange Book is the most up-to-date because there are updates made daily including generic drug.
Codes beginning with A signify the product is deemed therapeutically equivalent to the reference product for the category. The first letter -- A or B -- indicates whether the drug is therapeutically equivalent to other pharmaceutically equivalent products. Pharmaceutical Equivalents are drug products which contain the same active ingredients in the same strength and.
_ GITAM Institute of Pharmacy. Orange Book No TE code. View details PDF 949 KB.
The Orange Book has long been a reliable resource for information about FDA-approved drugs. There are broadly two types of therapeutic equivalent codes A-rated and B-rated drugs or codes.

Orange Book And Its Applications Legal Advantage
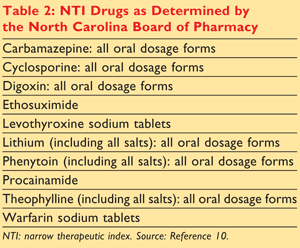
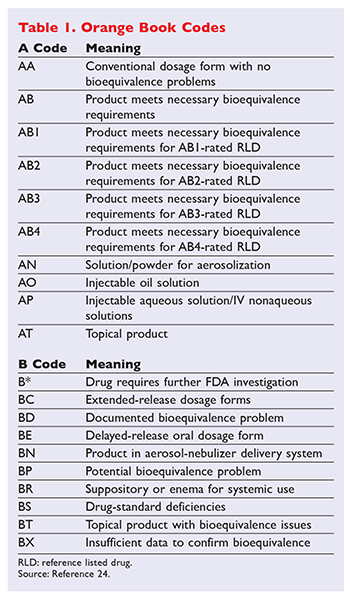
Generic Substitution Of Narrow Therapeutic Index Drugs
Orange Book And Its Applications Legal Advantage

A New Social History Of Pharmacy Pharmaceuticals Festival American Institute Of The History Of Pharmacy

Food And Drug Administration Fda U S Government Bookstore